Use case
The Polish Medical Research Agency has invested over €100 million to establish 19 Regional Digital Medicine Centres (RDMCs), positioning Poland as a global leader in digital medicine. These RDMCs provide advanced infrastructure for securing data, driving clinical research, and applying AI, aiming to transform medical research, patient care, and healthcare innovation through access to vast, reliable, and controlled data sources.
Problem addressed
The establishment of RDMCs in Poland addresses the challenge of digitising, standardising, and securely managing vast amounts of patient data across hospitals, universities, and biobanks to support advanced clinical research and improve healthcare outcomes. This multidimensional harmonisation of data will catalyse breakthroughs, especially in the field of precision medicine.
Added value
Improving the quality of medical data through standardisation and interoperability by establishing robust data-sharing standards.
Fostering cross-border medical research and strengthening international scientific collaboration.
Enabling secondary data analyses for scientific and public health advancements through the facilitation of big data analysis.
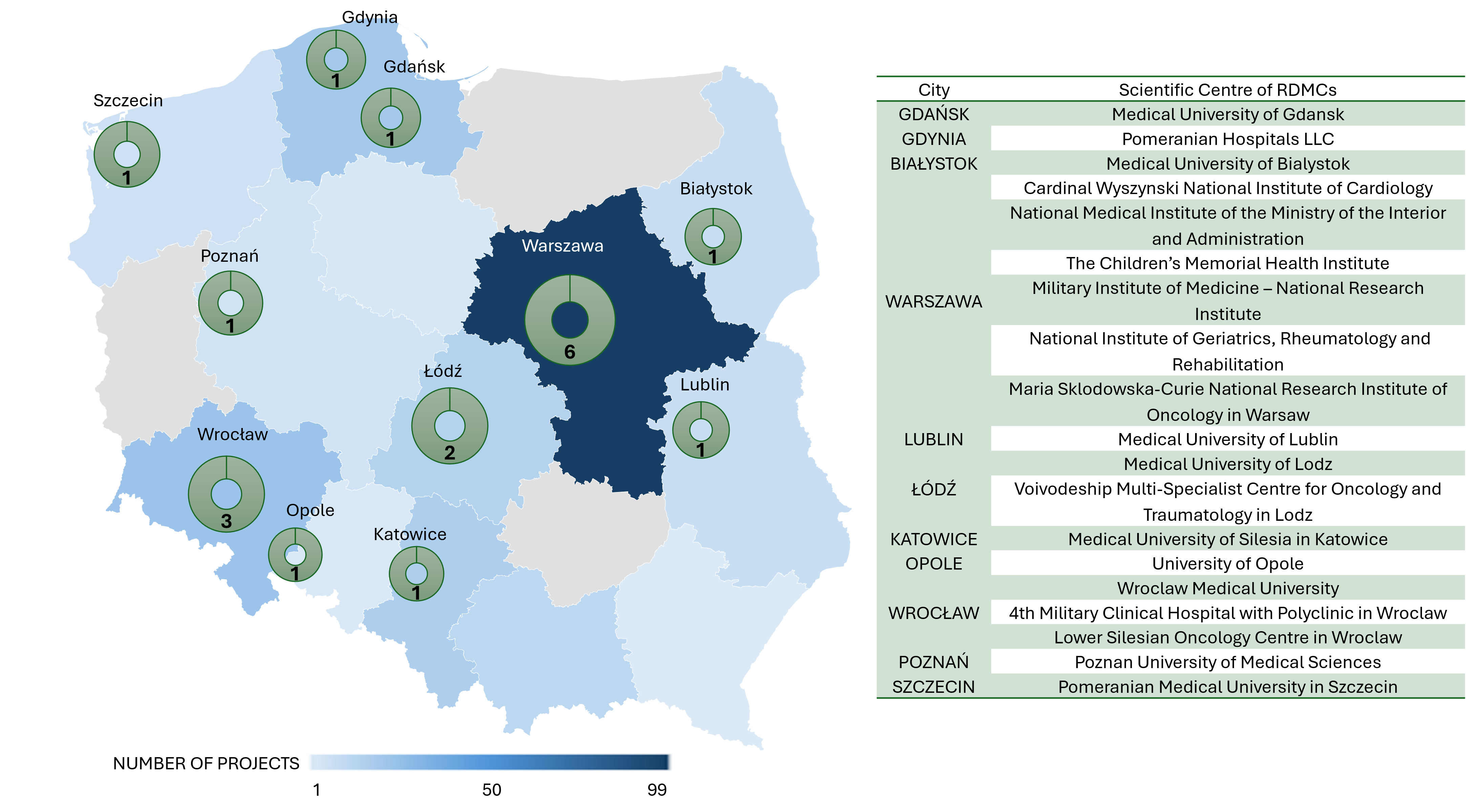
Geographical distribution of RDMCs, accompanied by a detailed description of their units. Figures provided by the Medical Research Agency (MRA) specifically for the purposes of the EOSC Macro-Roadmap. All rights reserved. Reproduction or distribution requires permission from the MRA.

